Fda Medical Device User Fees 2024. Food and drug administration (fda) issued a federal register notice announcing medical device user fees and annual establishment registration fees. Food and drug administration (fda) announced the medical device user.
For its fiscal year of 2024, stretching from october 1 2023 through september 30 2024, the food and drug administration (fda) medical device registration fee rates. This document addresses the plan for implementation and.
Fda, Fy 2024 Medical Device User Fees Notice.
The fda relies on user fees to fund the review and approval of medical devices.
“Without The Ability To Collect And Spend User Fees, The Review Time For Medical.
Food and drug administration (fda) unveiled the latest medical device user fees and annual establishment registration fees, which are set to take effect.
From 2023 To 2027, The Bill Would Allow The Fda To Collect $1.78 Billion To $1.9 Billion In User Fees Specifically For Medical Devices.
Images References :
 Source: vantagemedtech.com
Source: vantagemedtech.com
FDA Medical Device User Fees (MDUFA) for 2024 Vantage Medtech, From 2023 to 2027, the bill would allow the fda to collect $1.78 billion to $1.9 billion in user fees specifically for medical devices. The increase in fda user fees from fy 2023 to fy 2024 was 9.5%, except the annual fda registration fee, which increased by 17.87% to $7,653.
 Source: operonstrategist.com
Source: operonstrategist.com
FDA Announces New Medical Device User Fee Amendments ( MDUFA ) for FY, Medical device user fee amendments (mdufa) linkedin. This document addresses the plan for implementation and.
 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
FDA User Fees for FY 2024 released on July 28, 2023 Medical Device, Food and drug administration (fda) posted a federal register notice announcing the user fees for fiscal year 2024. “without the ability to collect and spend user fees, the review time for medical.
 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
FDA User Fees Archives Medical Device Academy Medical Device Academy, This document addresses the plan for implementation and. Select updates for the medical device user fee small business qualification and certification guidance draft guidance for industry and.
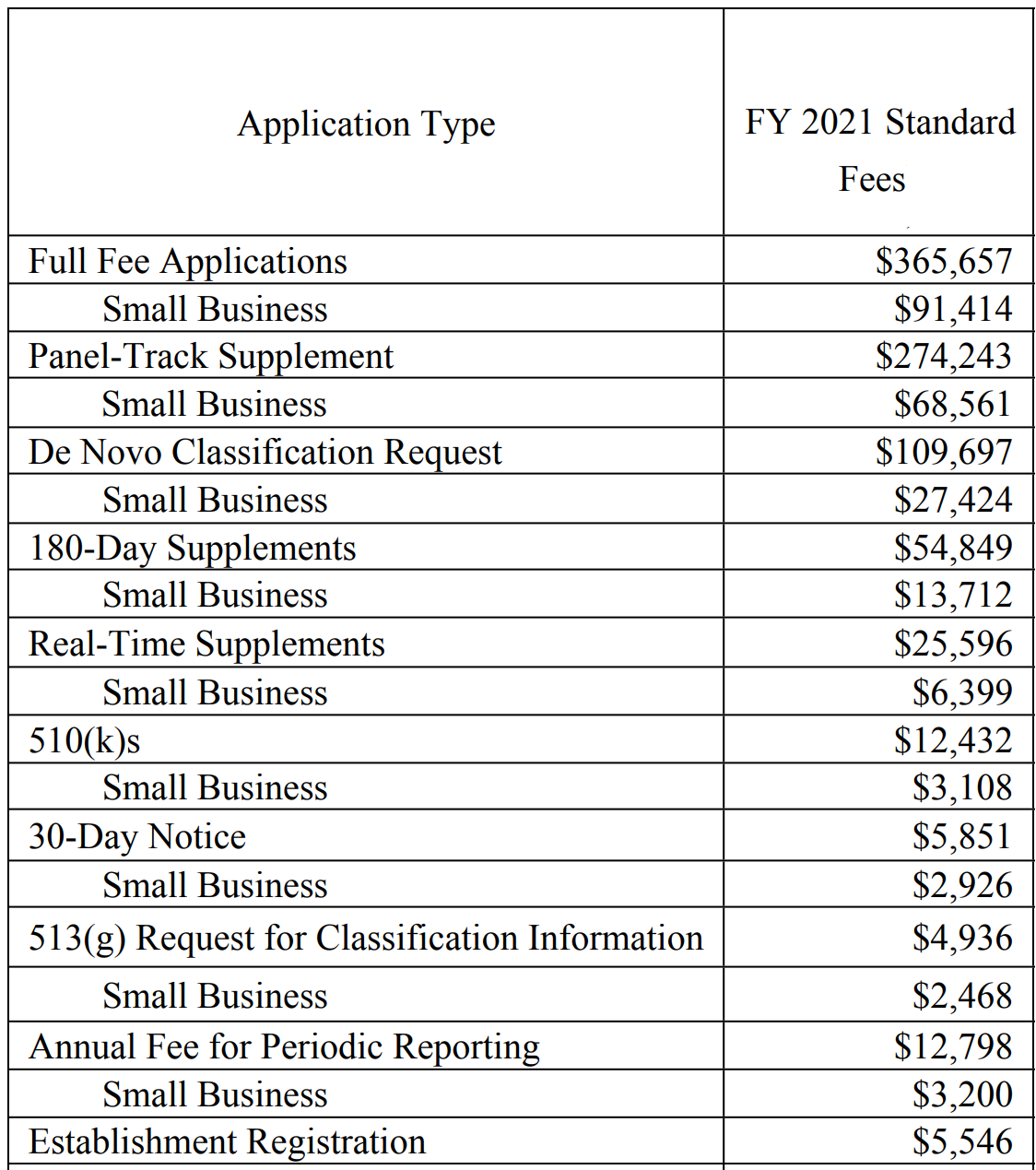 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
Medical Device 510k submissions, quality systems and training Medical, From 2023 to 2027, the bill would allow the fda to collect $1.78 billion to $1.9 billion in user fees specifically for medical devices. There are no waivers or reductions for small establishments, businesses, or groups in fy 2024.
 Source: emmainternational.com
Source: emmainternational.com
Understanding Medical Device User Fees, Medical device user fee amendments (mdufa) linkedin. For its fiscal year of 2024, stretching from october 1 2023 through september 30 2024, the food and drug administration (fda) medical device registration fee rates.
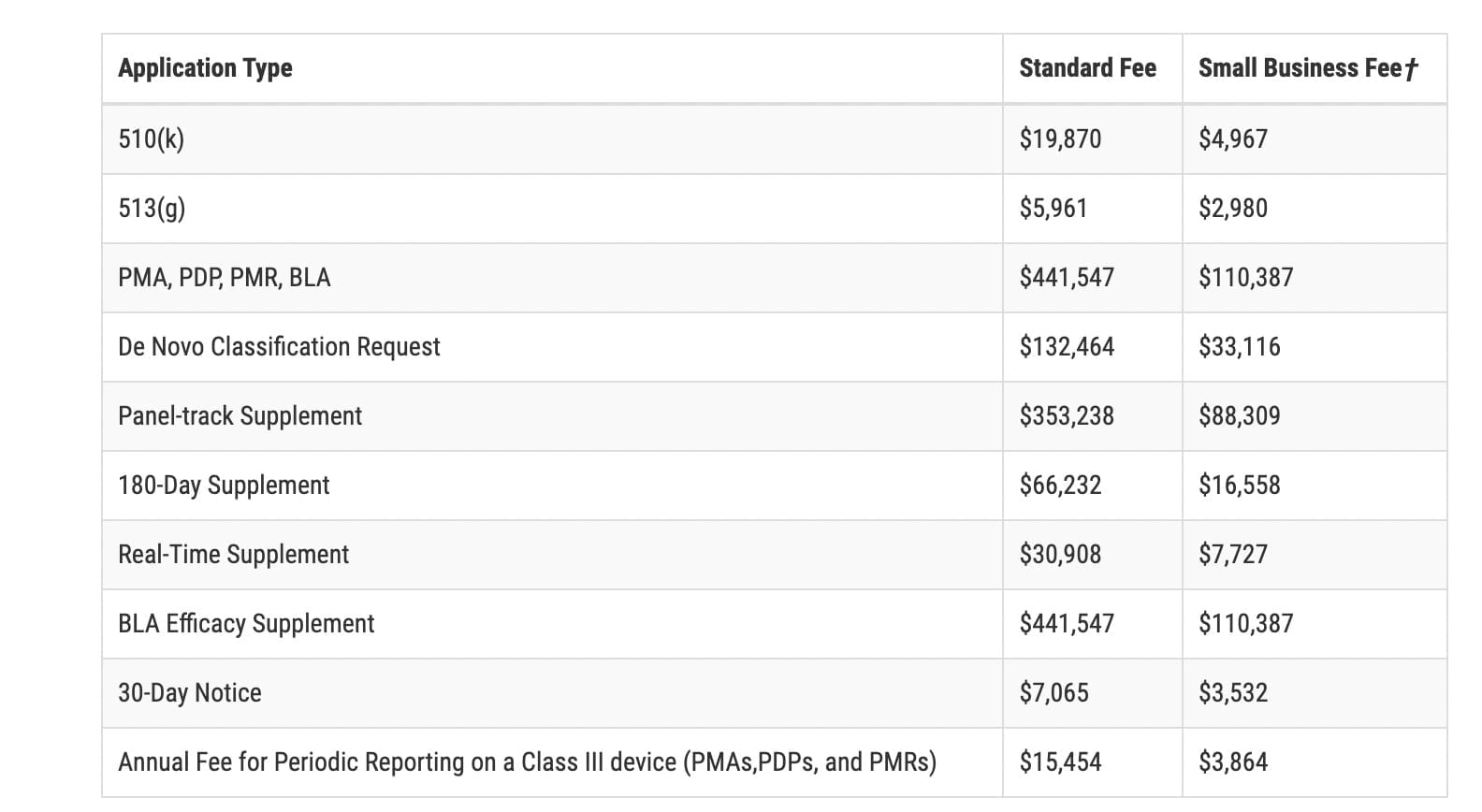 Source: cosmereg.com
Source: cosmereg.com
FDA 2023 Medical Device User Fees Announcement Cosmereg, From 2023 to 2027, the bill would allow the fda to collect $1.78 billion to $1.9 billion in user fees specifically for medical devices. Food and drug administration (fda) has announced new medical device user fee amendments (mdufa) for fiscal year 2024.
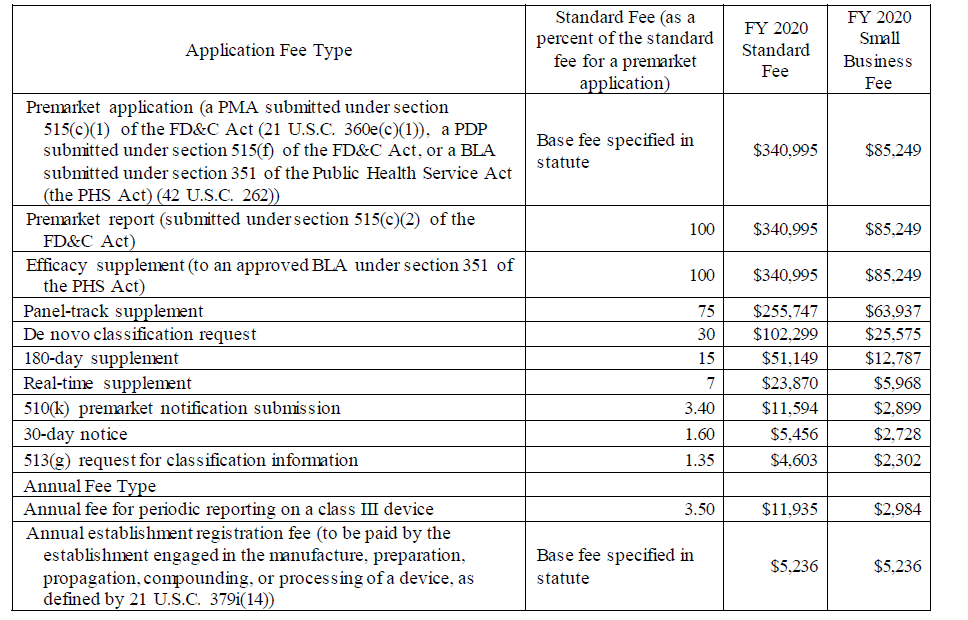 Source: www.lachmanconsultants.com
Source: www.lachmanconsultants.com
FDA Announces MDUFA and Outsourcing User Fee Rates for FY 2020 All, The increase in fda user fees from fy 2023 to fy 2024 was 9.5%, except the annual fda registration fee, which increased by 17.87% to $7,653. Along with the rule, the.
 Source: www.provisionfda.com
Source: www.provisionfda.com
FDA Medical Device User Fees Increase 7 for 2021 Fiscal Year, Food and drug administration (fda) posted a federal register notice announcing the user fees for fiscal year 2024. Select updates for the medical device user fee small business qualification and certification guidance draft guidance for industry and.
 Source: www.meditrial.net
Source: www.meditrial.net
US FDA publishes user fee amounts for medical device manufacturers in, “without the ability to collect and spend user fees, the review time for medical. User fees for fy 2024.
The Fda Announced The Fy 2024 Fda User Fees On July 28, 2023.
The increase in fda user fees from fy 2023 to fy 2024 was 9.5%, except the annual fda registration fee, which increased by 17.87% to $7,653.
The User Fees Reauthorized Include:
The prescription drug user fee act, the generic drug user fee act, the biosimilars user fee act, and the medical device user fee act.